Discussion on the sources and mechanism of supersaturated methane in euphotic seawater
-
摘要: 真光层海水中普遍存在甲烷过饱和现象,尤其是天然气水合物区真光层的甲烷明显异常。由于临近海气界面,真光层过饱和甲烷与大气甲烷排放及全球温室效应密切相关。目前,对真光层海水的过饱和甲烷来源仍没有统一的认识。综合前人研究成果梳理了真光层海水过饱和甲烷的来源,归纳了真光层海水过饱和甲烷现象形成的影响因素,进一步探讨了原位微生物可能参与的甲烷代谢机制。真光层过饱和甲烷可能来源于沉积物、临近河流或原位微生物,且受区域、季节、营养盐等多种因素的影响。由于受氧气影响,真光层海水甲烷产生的代谢机制有其特殊性,目前推测微生物可能依旧利用常规的产甲烷途径,它们存在于海水微厌氧环境中,或自身形成抵抗氧气影响的能力;此外,微生物也可能选择对氧不敏感的新的产甲烷途径。因此,针对天然气水合物区真光层甲烷过饱和现象,开展甲烷的来源和代谢机制的研究,以期为天然气水合物试采与开发的环境评价提供理论支撑,并为探究海水甲烷对大气及全球气候的影响提供理论依据。Abstract: Methane supersaturation occurs widely in the euphotic zone of oceans, especially in the areas with natural gas hydrate. It is closely related to atmospheric methane emission and global greenhouse effect due to the proximity of the sea-air interface. Up to date, it remains controversy concerning the source of supersaturated methane in euphotic seawater. This paper focuses on synthesizing the previous research results in order to sort out the sources of supersaturated methane, summarizing the influencing factors of supersaturated methane formation, and further exploring the mechanism of methane metabolism that in-situ microbes may participate in. The sources of supersaturated methane in euphotic zone may be transported from sediments, near-rivers or generated by in-situ microbes, and affected by various factors such as region, season, nutrient, and biological activities. Due to the influence of oxygen, the particularity of methanogenic mechanism is showed in euphotic seawater. Currently, it is speculated that conventional methanogenic pathways may be still performed by microorganisms, which exist in the micro-anaerobic environment of seawater, or generate the ability of resistance to oxygen; in addition, microorganisms may also choose new methanogenic pathways that are not sensitive to oxygen. Therefore, for the methane supersaturation phenomenon in euphotic seawater in natural gas hydrate area, the study of the sources and metabolic mechanisms of methane was carried out. It was hoped to provide theoretical support for the environmental assessment of gas hydrate test mining and development, and provide a theoretical basis for exploring the impact of seawater methane on the atmosphere and global climate.
-
Keywords:
- euphotic zone /
- supersaturated methane /
- methane sources /
- methanogenic archaea
-
东海陆架盆地位于欧亚板块、太平洋板块和印度洋—澳大利亚板块衔接处,其显生宙以来的大地构造位置属华夏构造域与特提斯构造域和西太平洋构造域的重叠区间[1-4](图1)。
东海陆架盆地南部地区,南与珠江口盆地相接,北与东海陆架盆地中北部毗邻,西靠浙闽隆褶带,东邻钓鱼岛台湾隆褶带,水深小于150 m,以海底地势平坦、宽度大为特点,由台西盆地、观音凸起、晋江凹陷、新竹凹陷、九龙江凹陷、澎湖—北港隆起等几个地质构造单元组成[5-8]。邻近陆域包括福建东南部以及广东东北部的广大地区。
东海陆架盆地及其邻区陆域中生代经历多期构造运动叠加,一些学者认为,该区晚三叠世—早侏罗世属于被动大陆边缘[7],而有些学者通过构造物理模拟等手段,恢复了该区构造演化历史,认为晚三叠世—早侏罗世属于主动大陆边缘,发育了大型坳陷盆地[2]。
自20世纪70年代起,我国石油勘探公司以及一些外国石油公司(在合作区块内)先后在东海南部开展钻探,据不完全统计,整个研究区及其邻近周边共钻井30余口。近年来,通过钻井地层、地震剖面解释及邻近陆域野外地质调查,建立了东海陆架盆地中生代地层序列(图2),认为东海陆架盆地发育上三叠统、中下侏罗统(福州组)、白垩系(厦门组、渔山组、闽江组、石门潭组下段)。前中生界基底主要为古生界或前寒武系岩系[9-11]。
东海陆架盆地是我国近海最大的中—新生界叠合含油气盆地之一,具有良好的油气勘探前景,是我国油气勘探开发重要的接替区[12-13]。其中,新生界的勘探已经取得了巨大的成功[14-17],而随着近些年来勘探技术水平的提升,中生界已然成为下一步油气勘探突破的新层系[5, 9, 12, 18-21]。
目前,东海陆架盆地南部钻井资料有限,揭示沉积古环境的资料亦有限,难以较客观全面地认识中生界发育状况。这就有必要开展与之具有相同大地构造环境中的邻近陆域沉积古环境的研究,由陆及海,从而推测东海陆架盆地南部沉积古环境,为后续开展海域中生界油气地质条件研究提供依据。
1. 陆域上三叠统—下侏罗统野外剖面特征
项目组近年来实施了多次陆地野外地质考察,考察地点包括粤东北及福建大部分地区(图3),主要考察对象为陆域中生界,其目的主要是通过观察陆域中生界典型露头及剖面,开展沉积古环境研究,进而推测东海陆架盆地南部中生代沉积古环境,为后续开展海域中生界油气地质条件研究提供依据。
1.1 海丰青年水库剖面(T3-J1)
该剖面位于广东省东南部海丰县境内青年水库附近。剖面地层为上三叠统头木冲组(T3t)、下侏罗统银瓶山组(J1y)及上龙水组(J1s),其中,下侏罗统银瓶山组整合接触于上三叠统头木冲组。
上三叠统头木冲组(T3t)总体沉积环境表现为水体震荡加深的滨海—浅海陆棚—深水陆棚环境。岩性主要发育波状、中层肉红色粉砂岩与灰黑色泥页岩不等层互层,以前者为主,表现出重力流沉积特征(图4a);局部发育多岩类,包括中—薄层、泥质粉砂岩、粉砂质泥岩,岩性频繁交互,厚度稳定,中间主体为一套具波状交错层理的中砂岩,似具牵引流特征,其底部发育灰黑色碳屑(泥粒)层,成层性好,连续发育,体现海平面波动,造成环境变化(图4b)。
下侏罗统银瓶山组(J1y)总体沉积环境表现为较深水环境,属于深水陆棚沉积。岩性主体为薄层—纹层肉红色粉砂质泥岩与浅灰色粉砂质泥岩互层,夹少许粉砂岩,波痕、交错层理发育,发育黄铁矿、碳屑等,为深水陆棚沉积(图5a),局部发育含量丰富、大小悬殊、扁平状泥粒、碳屑的细砂岩,表现为侧向迁移、垂向叠加的潮汐水道特征(图5b)以及厚度巨大的砂坝相细—中粒长石石英砂岩。
1.2 新丰梅坝—水口剖面(T3-J1)
该剖面位于广东省韶关市新丰县梅坝-水口沿河位置。剖面地层为上三叠统小水组(T3x)、下侏罗统麻笼组(J1m)。下侏罗统麻笼组整合接触于上三叠统小水组。
上三叠统小水组(T3x)总体沉积环境表现为水体震荡加深的滨海—浅海陆棚环境。岩性以中—厚层细砂岩、细粒长石石英砂岩为主(图6a),内部发育中—厚层透镜状砂岩与薄层粉砂岩不等厚互层,为滨海砂坝相(图6b),以及深灰色薄层粉砂岩与粉砂质泥岩互层,为浅陆棚相,常见贝壳类化石(图6c)及虫孔构造(图6d)。
下侏罗统麻笼组(J1m)发育厚层、块状,深灰色、灰黄色、灰白色石英(长石)砂岩,为风成滨岸沙丘,以及厚层、块状,致密坚硬细粒(长石)石英砂岩,为砂坝相沉积。
1.3 南靖油柑坪剖面(T3w)
该剖面位于福建省漳州市南靖县西部。剖面地层为上三叠统文宾山组上段(T3w2)。
上三叠统文宾山组(T3w)整体属于三角洲相沉积。上段下部发育水平层理、透镜层理、脉状层理、槽状交错层理等,表明属三角洲前缘亚相的远砂坝—河口砂坝微相(图7a);上段中部具向上变细的正粒序,粉砂岩发育水平层理、波状层理,局部含植物化石碎片,其沉积相为三角洲平原亚相局部,夹少量炭质泥岩,为受湖侵影响的滨湖相沉积;上段上部沉积物以含砾粗砂岩、中粗粒砂岩为主,向上变为砂质泥岩、细(杂)砂岩,砾石磨圆度差,岩石成分、结构成熟度较低,反映了快速堆积、水动力较强特点,具向上变细、细碎屑岩厚度大的河流相沉积层序结构特征,故上部应为三角洲平原分流河道微相(图7b)。
1.4 漳平高明坑剖面(J1l)
该剖面位于福建省龙岩市漳平县城关南约5.3 km。剖面地层为下侏罗统梨山组(J1l),层序较全,顶底完整。
下侏罗统梨山组整体表现为陆相沉积环境。岩性组合上包括多套砾岩层叠加覆盖于下部地层,砾岩层底界清晰,冲刷面特征典型,为冲积扇相的河道亚相沉积(图8a);细砂岩、粉砂岩、泥岩和煤层互层,为辫状河道和辫状分流平原环境(图8b);灰黄色粉砂岩和黑色煤层、黑色碳质泥页岩互层,为沼泽相(图8c);中—厚层灰黄色中粒石英砂岩,为滨湖砂坝微相沉积(图8d)。
2. 岩石样品地球化学分析测试
对野外采集样品(表1)进行常量、微量元素分析,其中,采用电感耦合等离子体质谱仪(ICP-MS)开展微量元素分析,采用X射线荧光光谱仪(XRF)开展主量元素分析。野外样品“MK”采自于新丰梅坝—水口剖面,“QN”采自于海丰青年水库剖面,部分样品分析测试结果见表1。
表 1 常量、微量元素分析测试结果(部分)Table 1. Analysis results of major and trace element(a part)样品编号 Mg
/%Si
/%Al
/%Ca
/%Fe
/%K
/%Na
/%Ba
/10−6Ni
/10−6Th
/10−6U
/10−6V
/10−6Sr
/10−6MK-XS-03(1) 0.88 22.77 7.30 0.48 6.17 0.86 0.80 564 11 0.4 1.0 60 190 MK-XS-05 0.60 27.70 1.94 0.17 0.60 0.31 0.06 45 6 0.1 1.6 20 5 MK-XS-06 1.49 13.90 2.67 0.37 6.74 1.10 0.61 430 14 0.5 1.8 49 175 MK-XS-14(2) 0.32 17.92 2.85 0.20 3.71 0.35 0.04 39 5 0.2 0.4 14 3 MK-XS-15 0.64 15.82 4.72 0.50 5.25 1.13 0.26 443 34 0.6 3.1 70 12 MK-XS-27 0.73 15.79 6.21 0.97 13.47 2.76 0.55 260 88 3.4 10.2 206 75 MK-XS-36 1.37 26.41 11.22 0.32 2.23 1.56 0.59 700 13 0.5 0.3 54 279 MK-XS-37 0.49 19.19 6.38 0.33 3.38 0.93 0.56 283 5 0.4 0.8 36 62 MK-XS-38 1.18 24.25 9.24 0.50 7.51 1.11 0.51 516 25 0.8 1.9 85 147 MK-XS-40 0.86 25.62 8.95 0.57 9.61 1.94 0.25 195 64 1.9 4.2 194 12 MK-XS-41 1.30 24.56 8.33 0.44 8.56 1.01 0.30 413 26 0.7 1.9 97 17 QN-TMC-03 0.85 21.88 10.93 0.19 1.76 1.08 0.41 651 3 0.4 0.5 21 52 QN-TMC-04 0.74 22.48 7.74 0.83 13.00 1.32 0.57 514 47 1.5 3.5 129 24 QN-TMC-06 0.57 26.80 8.54 0.25 4.45 1.91 0.91 710 13 0.4 1.3 33 46 QN-YPS-16(2) 1.06 25.08 10.63 0.65 8.29 2.18 0.58 947 34 1.0 2.2 48 193 QN-YPS-20 1.25 27.72 12.05 0.54 8.35 2.03 0.64 719 26 0.6 1.8 58 70 2.1 古气候分析
通常,Sr/Cu比值为1.3~5.0指示温湿气候,该值大于5.0则指示干热气候。有的学者也提出Sr/Cu的值为1~10指示温湿气候,大于10指示干热气候[22]。Sr/Ba比值常用来区分淡水和咸水沉积环境。水动力条件变化较大的滨海和浅海地带,大量的Sr离子通过以生物堆积作用为主的方式沉淀下来,形成较高的Sr/Ba比值(Sr/Ba>1)。一般来说,Sr/Ba>1时指示的是咸水沉积环境[22]。
根据测试结果(表2)可知,研究区Sr/Cu比值为0.32~50.50,平均为9.67,除个别样品(MK-XS-03(1)、MK-XS-06、MK-XS-36)Sr/Cu值大于10以外,绝大部分样品Sr/Cu值小于10,反映出研究区样品形成于温湿气候条件下。3个Sr/Cu值大于10的样品表明形成于干热气候条件下,推测此时水体蒸发量较大而供给不足,导致水体变浅咸化,这点可以从水体古盐度证明,3个样品的古盐度要略高与其余样品(图9)。
表 2 福建东南部地区岩石地球化学测试结果(引自文献[25])Table 2. Geochemical testing results of samples from southeastern Fujian(from reference [25])序号 采样地点 采样层位 Ga/(ug/g) Th/(ug/g) U/(ug/g) Ba/(ug/g) B/(ug/g) Ni/(ug/g) Sr/(ug/g) V/(ug/g) 1 福建云霄西部 上三叠统文宾山组 26.01 15.10 3.91 667.3 84.74 38.86 40.15 102.7 2 24.09 13.49 4.10 722.7 28.66 47.46 161.7 85.57 3 24.08 5.53 1.03 248.2 3.74 29.29 289.8 271.6 4 24.88 14.04 2.72 636.4 8.96 23.88 33.24 117.4 5 29.14 17.54 3.66 596.5 90.03 17.34 98.79 128.5 6 31.90 15.34 3.05 785.1 15.70 9.21 151.4 135.0 7 漳平钱坂 下侏罗统梨山组 14.40 12.68 2.50 407.8 67.58 4.96 14.02 49.45 8 15.43 16.65 3.46 455.5 45.97 12.09 19.09 59.44 9 15.74 9.39 2.16 659.3 21.31 11.87 19.77 43.83 2.2 沉积水介质氧化—还原条件分析
通常判断沉积水体的氧化还原条件主要依据同生矿物组合。常用的恢复水体氧化条件的地球化学指标主要有V/(V+Ni)值、Ni/Co值、V/Ni值、V/Cr值、Cu/Zn值以及U/Th值等。
V/Ni和V/(V+Ni)比值:V和Ni同属铁族元素,其离子价态易随环境的氧化还原程度变化而变化,V和Ni容易被胶体质点和黏土等吸附沉淀,V更易于在氧化环境下被吸附而富集,而Ni则在还原环境下更易吸附富集,因此,元素的V/(V+Ni)比值可反映沉积水体的氧化还原环境。通常V/(V+Ni)> 0.54代表厌氧环境,V/(V+Ni)比值为0.46~0.60表示贫氧沉积环境,V/(V+Ni)< 0.46指示富氧沉积环境[22]。
研究区样品V/(V+Ni)为0.58~0.88,平均值为0.76,表明其还原性较强,处于缺氧的沉积环境,有利于有机质保存(图10)。
Hatch等[23]对北美堪萨斯州上宾夕法尼亚系黑色页岩的研究表明,V/(V+Ni)值能有效反映环境氧化还原条件,高的V/(V+Ni)值(0.84~0.89)反映水体分层,底层水体出现H2S的厌氧环境;中等比值(0.54~0.82)为水体分层不强烈的厌氧环境;低值(0.46~0.60)为水体分层弱的贫氧环境。
铀元素在自然界水体中因易与还原剂作用生成铀黑或其他物质吸附而沉淀;钍的络合物在弱碱性溶液中易水解,变成氧化物或氢氧化物沉淀。一般U/Th<0.75指示氧化环境,0.75~1.25为贫氧环境,U/Th>1.25为厌氧环境[24]。
研究区U/Th值为0.31~11.53,仅有一个样品处于贫氧环境,其余样品均为厌氧环境(图10)。
3. 讨论
3.1 晚三叠世沉积古环境
在南部的粤东地区,上三叠统野外剖面特征及典型岩相组合表现为,岩性复杂,以中—厚层细砂岩、细粒长石石英砂岩为主,泥质粉砂岩、粉砂质泥岩,岩性频繁交互,厚度稳定。沉积构造丰富多样,以低角度槽状交错层理为主,波痕发育,常见贝壳类化石及虫孔构造,符合陆棚相沉积环境特征。
新丰梅坝—水口上三叠统小水组碎屑岩矿物以石英矿屑、岩屑为主,结构成熟度高,分选好,磨圆中等,含相当数量的白云母矿屑和少量的铁质矿物颗粒,且颗粒具有较明显的定向性(图11),表明当时的沉积环境属于水动力条件较好的较深水沉积环境。
岩石样品地球化学测试结果表明,粤东地区晚三叠世处于温湿气候环境,沉积水体深度较大,为厌氧沉积环境。
综合上述特征认为,粤东地区晚三叠世时期,气候温暖湿润,总体表现为水体震荡加深的滨海—浅海陆棚—深水陆棚环境。
在北部的福建漳州南靖地区,上三叠统野外剖面特征及典型岩相组合表现为,岩性较细,以砂岩为主,泥质含量偏低,砂岩层内部泥质夹层少,偶见泥岩为炭质,发育水平层理、透镜层理、脉状层理、槽状交错层理等,表明属三角洲前缘亚相的远砂坝—河口砂坝微相,局部发育的含砾粗砂岩、中粗粒砂岩砾石磨圆度差,岩石成分、结构成熟度较低,反映了快速堆积、水动力较强特点,为三角洲平原分流河道微相。
福建地质调查研究院2009年对漳州云霄地区开展元素地球化学调查[25]发现(表2),该地区晚三叠世时期,沉积岩中硼的含量在不同地区和不同环境下,含量相差甚远;Sr/Ba比大部分小于0.2,少部分大于1;U/Th比均小于0.2,证明该区在晚三叠世总体属于陆相沉积环境,但受到海侵的影响。
综合上述特征认为,福建东南部晚三叠世时期为受海侵作用影响的海陆过渡相环境,发育河流三角洲体系、河流-湖泊体系和碎屑滨岸体系等。
横向对比南部的粤东地区和北部的福建东南部地区不难发现,晚三叠世表现为南海北陆的特点,南部发育海相沉积,北部发育海陆过渡相沉积,海侵方向来自南部。
3.2 早侏罗世沉积古环境
早侏罗世,基本继承了晚三叠世以来的沉积格局,在南部的粤东地区,下侏罗统野外剖面特征及典型岩相组合表现为下细上粗的反旋回特征,下部沉积环境稳定,发育岩性单一,表现为薄层粉、细砂岩与泥岩韵律互层,为半深海沉积环境,向上砂岩粒度变粗,砂泥比增大,发育楔状、波状、槽状等层理,为滨海砂坝沉积。总体沉积格局为浅水陆棚—深水陆棚相。
海丰青年水库下侏罗统银瓶山组发育多种岩类岩相,细粒岩石中矿物颗粒以次圆为主,含不等量的次棱角,结构成熟度和成分成熟度一般—中等(图12),表明当时的沉积环境属于近水贫物源的较深水环境。
岩石样品地球化学测试结果表明,粤东地区早侏罗世处于温湿气候环境,沉积水体深度较大,为厌氧沉积环境。
综合上述特征认为,粤东地区早侏罗世时期,气候温暖湿润,总体表现为水体较深的浅海陆棚—深水陆棚环境。
在北部的福建龙岩漳平地区,下侏罗统野外剖面特征及典型岩相组合表现为,下部岩性以砾岩、粗砂岩、中砂岩为主,向上粒度逐渐变细,以粉砂岩、泥质粉砂岩、粉砂质泥岩和黑色泥岩为主,垂向上表现为冲积扇—河道—沼泽—滨湖环境。
福建地质调查研究院2009年对漳平钱坂地区开展元素地球化学调查[25]发现(表2),该地区早侏罗世时期,各指标差异较大,可能受后期成岩作用及强烈的岩浆活动影响。沉积岩中硼的含量在不同地区和不同环境下,含量相差甚远;Sr/Ba比小于0.1;U/Th比均小于0.2,证明该区在晚三叠世总体属于受海侵影响的陆相碎屑滨岸沉积体系。
综合上述特征认为,福建东南部早侏罗世时期为受海侵作用影响的陆相碎屑滨岸沉积环境,发育冲积扇、河道、沼泽、滨湖等沉积相类型。
横向对比南部的粤东地区和北部的福建东南部地区,早侏罗世基本继承了晚三叠世以来的沉积格局,表现为南海北陆的特点,海侵方向来自南部(图13)。
4. 结论
(1)东海陆架南部邻近陆域晚三叠世—早侏罗世时期,属于温湿气候环境,由于沉积水体深度较大,大部分地球化学测试样品指示厌氧环境。
(2)晚三叠世,在南部的粤东地区,主要表现为水体震荡加深的滨海—浅海陆棚—深水陆棚环境,局部地区发育成层性很好的、波状起伏的沉积岩地层,表现出深水重力流的沉积特征;在北部的福建漳州南靖地区,主要表现为受海侵作用影响的海陆过渡相环境,发育河流三角洲体系、河流-湖泊体系和碎屑滨岸体系等。
(3)早侏罗世基本继承了晚三叠世以来的沉积格局,在南部的粤东地区,主要表现为水体较深的深水陆棚环境,发育深水陆棚体系、滨海砂坝等;在北部的福建龙岩漳平地区,主要表现为海陆过渡相—陆相沉积环境,发育河流体系、河流三角洲体系和滨湖沉积体系等。
(4)纵向上,晚三叠世海侵达到高潮,之后出现短暂海退,沉积物也相应变粗,至早侏罗世又发生新一期的海侵;横向上,整个东海陆架南部邻近陆域水体沿浙闽古隆起地势低洼处自南向北水体逐渐变浅,表现为南海北陆的特点,海侵方向来自南部。
致谢:本次研究中的岩石样品地球化学测试工作由中国地质大学(北京)承担,同时,野外工作得到中国地质大学(北京)王海荣副教授及福建省地质调查研究院徐立明高级工程师的支持和指导,最后,王星星博士对文章进行了核对,在此一并表示感谢。
-
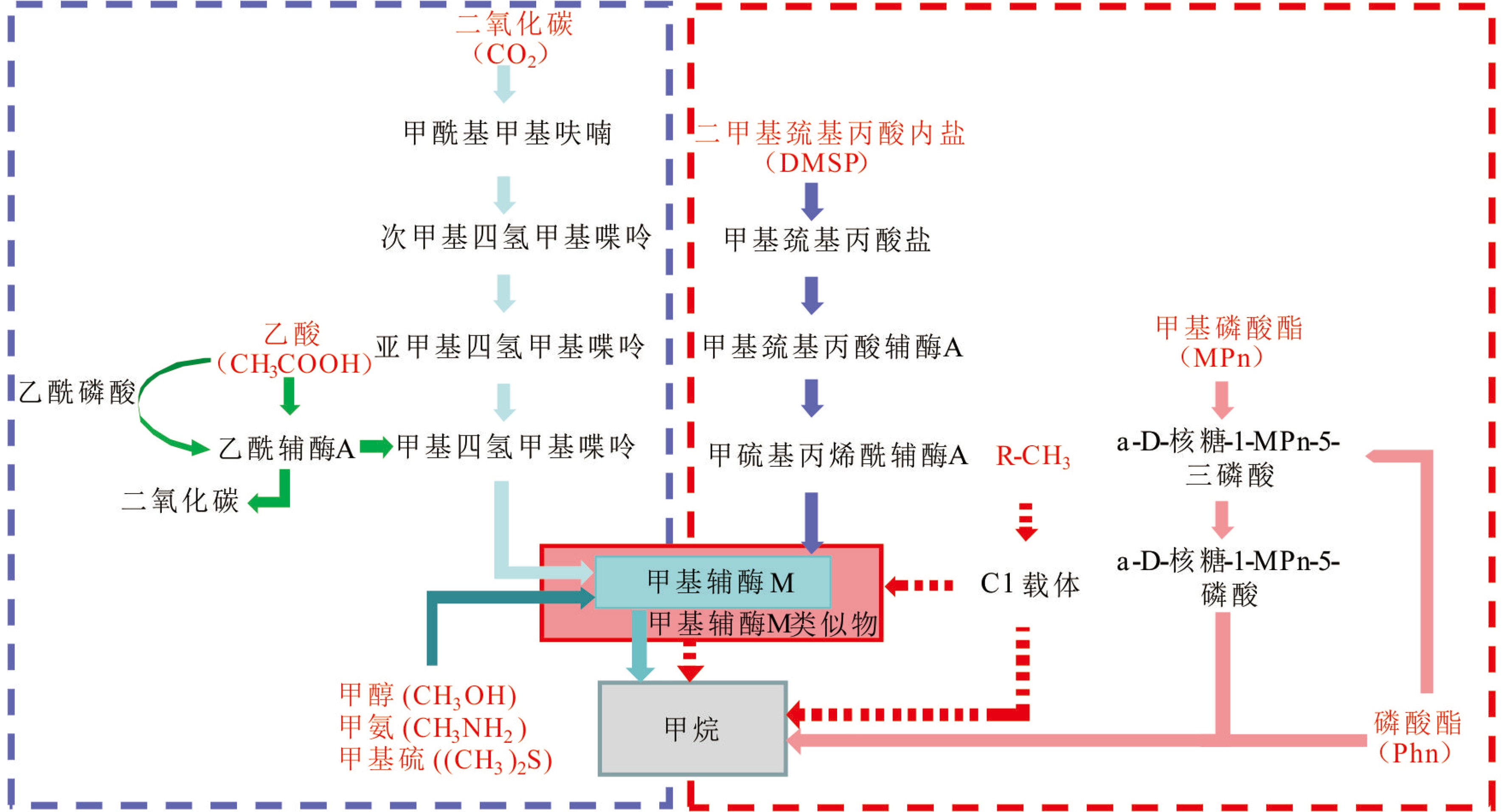
图 2 真光层海水甲烷产生可能存在的代谢途径(据文献[95,10,106]修改)
虚线蓝框表示传统产甲烷途径,包括CO2还原途径、甲基裂解途径和乙酸发酵途径;虚线红框表示替代的新产甲烷途径,包括C-S键裂解途径,C-P键裂解途径,以及以R-CH3为底物的假设途径(红色虚线箭头)。每种代谢途径的底物用红色字体标出。
Figure 2. Possible methanogenesis metabolic pathways in ocean’s euphotic zone (modified from references [95,10,106])
The dash blue box indicates the classical methanogenic pathways mediated by methanogenic archaea, including the CO2 reduction pathway, the methylation pathway and the acetate fermentation pathway; the dash red box indicates bacterial-mediated alternative new pathways, including cleavage pathway of the C-S bond, cleavage pathway of the C-P bond and hypothetical pathway with R-CH3 as a substrate (dash red arrows). The substrates for each metabolic pathway are indicated in red.
表 1 真光层海水甲烷过饱和现象的实例
Table 1 Examples of methane supersaturation in euphotic zone of the ocean
位置 海域甲烷分布 真光层过饱和甲烷浓度 参考文献 东、黄海 春季表层海水中溶解甲烷浓度自沿岸向外海里呈逐渐降低趋势,在长江口外甲烷浓度呈明显的舌状分布 黄海表层甲烷春季为3.43±0.23 nM;东海表层甲烷
春季为3.24±0.59 nM,夏季为12.8±14.0 nM[27] 南海北部 从珠江口到外海表层海水甲烷浓度逐渐降低 表层甲烷浓度为2.4~5.9 nM,过饱和度达134%~297% [26] 易北河口及相邻的
北海甲烷分布受河口富甲烷输入和外海贫甲烷稀释的
共同影响海岸附近的甲烷平均浓度为30±13 nM、外海浓度为
14±6 nM[28] 南海北部陆坡天然气水合物区 不同季节,表层海水甲烷浓度变化较大 部分季节上层100 m甲烷浓度过饱和,高达20~30 nM [9] 亚热带北太平洋 表层海水甲烷浓度与季节无明显关系 表层海水(<300 m)甲烷浓度2~3 nM,
甲烷通量达到1.6 μmol·m−2·d−1[29] 北极洋大陆架西伯利亚东部海域 超过50%水域的表层海水甲烷过饱和 表层海水浓度范围是0~200 nM [22] 波罗的海中部哥特兰盆地 东部水域甲烷偏高,受极地水输入的影响 东部水域表层海水甲烷浓度与大气甲烷平衡(~3 nM),而浅层海水(20~40 m)浓度达15~77 nM [30] 极地陆架区域斯图尔峡湾 表层海水和次表层海水的甲烷互不影响 水体甲烷浓度范围是5~55 nM [31] 智利中部大陆架 甲烷浓度可能受风力驱动的沿海上升流影响 表层海水(0~30 m)甲烷饱和度为125%~550%
(O2饱和度>100%)[32] 弗拉姆海峡西部区域 寡营养的北部极地水输入,甲烷浓度偏高 水体浅表层(<20 m)表层甲烷浓度范围是6~9 nM [7] 黑海 研究区域位于冷泉区,甲烷浓度随深度逐渐升高 近表层甲烷浓度约为5 nM [5] 波罗的海 表层海水盐度偏低,从底层到表层溶解甲烷浓度
呈梯度下降表层甲烷浓度约为4 nM [6] 表 2 存活于有氧环境下产甲烷古菌类群
Table 2 The surviving methanogenic archaeal communities in oxic environments
产甲烷古菌类型 产甲烷古菌类群 参考文献 氢营养型 Methanomicrobiales(目) Methanogenium(科) [49, 54] Methanobacteriales(目) Methanobacterium(属), Methanobrevibacter(属) [49, 59] Methanocellales(目) Methanocella(属) [50-51, 54] 乙酸营养型 Methanosarcinales(目) Methanosaeta/Methanothrix(种) [52, 54] Methanosarcina(属) [50-51, 54] 甲基营养型 Methanosarcinales(目) Methanosarcinaceae(科) [54, 56] Methanosarcinales(目) Methanomicrococcus blatticola(种) [60] -
[1] ICPP. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change[M]. Cambridge: Cambridge University Press, 2013.
[2] Judd A G, Hovland M, Dimitrov L I, et al. The geological methane budget at Continental Margins and its influence on climate change [J]. Geofluids, 2002, 2(2): 109-126. doi: 10.1046/j.1468-8123.2002.00027.x
[3] Reeburgh W S. Oceanic methane biogeochemistry [J]. Chemical Reviews, 2007, 107(2): 486-513. doi: 10.1021/cr050362v
[4] Cicerone R J, Oremland R S. Biogeochemical aspects of atmospheric methane [J]. Global Biogeochemical Cycles, 1988, 2(4): 299-327. doi: 10.1029/GB002i004p00299
[5] Schmale O, Beaubien S E, Rehder G, et al. Gas seepage in the Dnepr paleo-delta area (NW-Black Sea) and its regional impact on the water column methane cycle [J]. Journal of Marine Systems, 2010, 80(1-2): 90-100. doi: 10.1016/j.jmarsys.2009.10.003
[6] Schmale O, Blumenberg M, Kießlich K, et al. Aerobic methanotrophy within the pelagic redox-zone of the Gotland Deep (central Baltic Sea) [J]. Biogeosciences, 2012, 9(12): 4969-4977. doi: 10.5194/bg-9-4969-2012
[7] Damm E, Thoms S, Beszczynska-Möller A, et al. Methane excess production in oxygen-rich polar water and a model of cellular conditions for this paradox [J]. Polar Science, 2015, 9(3): 327-334. doi: 10.1016/j.polar.2015.05.001
[8] 张桂玲, 张经. 海洋中溶存甲烷研究进展[J]. 地球科学进展, 2001, 16(6):829-835. [ZHANG Guiling, ZHANG Jing. Advances in studies of dissolved methane in seawater [J]. Advance in Earth Sciences, 2001, 16(6): 829-835. doi: 10.3321/j.issn:1001-8166.2001.06.012 [9] 梁前勇, 赵静, 夏真, 等. 南海北部陆坡天然气水合物区海水甲烷浓度分布特征及其影响因素探讨[J]. 地学前缘, 2017, 24(4):89-101. [LIANG Qianyong, ZHAO Jing, XIA Zhen, et al. Distribution characteristics and influential factors of dissolved methane in sea water above gas hydrate area on the northern slope of the South China Sea [J]. Earth Science Frontiers, 2017, 24(4): 89-101. [10] Tang K W, Mcginnis D F, Ionescu D, et al. Methane production in oxic lake waters potentially increases aquatic methane flux to air [J]. Environmental Science & Technology Letters, 2016, 3(6): 227-233.
[11] McGinnis D F, Kirillin G, Tang K W, et al. Enhancing surface methane fluxes from an oligotrophic lake: exploring the microbubble hypothesis [J]. Environmental Science & Technology Letters, 2015, 49(2): 873-880.
[12] Wolfe R S. Microbial formation of methane [J]. Advances in Microbial Physiology, 1971, 6: 107-146. doi: 10.1016/S0065-2911(08)60068-5
[13] Lamontagne R A, Swinnerton J W, Linnenbom V J, et al. Methane concentrations in various marine environments [J]. Journal of Geophysical Research, 1973, 78(24): 5317-5324. doi: 10.1029/JC078i024p05317
[14] Hinrichs K U, Boetius A. The anaerobic oxidation of methane: new insights in microbial ecology and biogeochemistry[M]//Wefer G, Billett D, Hebbeln D, et al. Ocean Margin Systems. Berlin, Heidelberg: Springer, 2002: 457-477.
[15] 尉建功, 杨胜雄, 梁金强, 等. 海洋钻探对甲烷渗漏的影响: 以南海北部天然气水合物钻探GMGS2-16站位为例[J]. 海洋地质与第四纪地质, 2018, 38(5):63-70. [WEI Jiangong, YANG Shengxiong, LIANG Jinqiang, et al. Impact of seafloor drilling on methane seepage—enlightenments from natural gas hydrate drilling site GMGS2-16, northern South China Sea [J]. Marine Geology & Quaternary Geology, 2018, 38(5): 63-70. [16] Boetius A, Wenzhöfer F. Seafloor oxygen consumption fuelled by methane from cold seeps [J]. Nature Geoscience, 2013, 6(9): 725-734. doi: 10.1038/ngeo1926
[17] Bastviken D, Cole J, Pace M, et al. Methane emissions from lakes: dependence of lake characteristics, two regional assessments, and a global estimate [J]. Global Biogeochemical Cycles, 2004, 18(4): GB4009.
[18] DelSontro T, Kunz M J, Kempter T, et al. Spatial heterogeneity of methane ebullition in a large tropical reservoir [J]. Environmental Science & Technology, 2011, 45(23): 9866-9873.
[19] Ostrovsky I, McGinnis D F, Lapidus L, et al. Quantifying gas ebullition with echosounder: the role of methane transport by bubbles in a medium‐sized lake [J]. Limnology and Oceanography: Methods, 2008, 6(2): 105-118. doi: 10.4319/lom.2008.6.105
[20] DelSontro T, McGinnis D F, Sobek S, et al. Extreme methane emissions from a Swiss hydropower reservoir: contribution from bubbling sediments [J]. Environmental Science & Technology, 2010, 44(7): 2419-2425.
[21] DelSontro T, McGinnis D F, Wehrli B, et al. Size does matter: importance of large bubbles and small-scale hot spots for methane transport [J]. Environmental Science & Technology, 2015, 49(3): 1268-1276.
[22] Shakhova N, Semiletov I, Salyuk A, et al. Extensive methane venting to the atmosphere from sediments of the East Siberian Arctic Shelf [J]. Science, 2010, 327(5970): 1246-1250. doi: 10.1126/science.1182221
[23] Schmale O, Haeckel M, McGinnis D F. Response of the Black Sea methane budget to massive short-term submarine inputs of methane [J]. Biogeosciences, 2011, 8(4): 911-918. doi: 10.5194/bg-8-911-2011
[24] Bastviken D, Ejlertsson J, Tranvik L. Measurement of methane oxidation in lakes: a comparison of methods [J]. Environmental Science & Technology, 2002, 36(15): 3354-3361.
[25] Kankaala P, Huotari J, Peltomaa E, et al. Methanotrophic activity in relation to primary and bacterial production in a boreal humic lake [J]. SIL Proceedings, 1922-2010, 2005, 29(1): 250-253. doi: 10.1080/03680770.2005.11902007
[26] Zhou H Y, Yin X J, Yang Q H, et al. Distribution, source and flux of methane in the western Pearl River Estuary and northern South China Sea [J]. Marine Chemistry, 2009, 117(1-4): 21-31. doi: 10.1016/j.marchem.2009.07.011
[27] 张桂玲. 中国近海部分海域溶解甲烷和氧化亚氮的生物地球化学研究[D]. 中国海洋大学博士学位论文, 2004. ZHANG Guiling. Studies on biogeochemistry of dissolved methane and nitrous oxide in the coastal waters of China[D]. Doctor Dissertation of Ocean University of China, 2004.
[28] Osudar R, Matoušů A, Alawi M, et al. Environmental factors affecting methane distribution and bacterial methane oxidation in the German Bight (North Sea) [J]. Estuarine, Coastal and Shelf Science, 2015, 160: 10-21. doi: 10.1016/j.ecss.2015.03.028
[29] Holmes M E, Sansone F J, Rust T M, et al. Methane production, consumption, and air-sea exchange in the open ocean: an evaluation based on carbon isotopic ratios [J]. Global Biogeochemical Cycles, 2000, 14(1): 1-10. doi: 10.1029/1999GB001209
[30] Schmale O, Wäge J, Mohrholz V, et al. The contribution of zooplankton to methane supersaturation in the oxygenated upper waters of the central Baltic Sea [J]. Limnology and Oceanography, 2018, 63(1): 412-430. doi: 10.1002/lno.10640
[31] Damm E, Kiene R P, Schwarz J, et al. Methane cycling in Arctic shelf water and its relationship with phytoplankton biomass and DMSP [J]. Marine Chemistry, 2008, 109(1-2): 45-59. doi: 10.1016/j.marchem.2007.12.003
[32] Florez-Leiva L, Damm E, Farías L. Methane production induced by dimethylsulfide in surface water of an upwelling ecosystem [J]. Progress in Oceanography, 2013, 112-113: 38-48. doi: 10.1016/j.pocean.2013.03.005
[33] Owens N J P, Law C S, Mantoura R F C, et al. Methane flux to the atmosphere from the Arabian Sea [J]. Nature, 1991, 354(6351): 293-296. doi: 10.1038/354293a0
[34] Tilbrook B D, Karl D M. Methane sources, distributions and sinks from California coastal waters to the oligotrophic North Pacific gyre [J]. Marine Chemistry, 1995, 49(1): 51-64. doi: 10.1016/0304-4203(94)00058-L
[35] Schulz M, Faber E, Hollerbach A, et al. The methane cycle in the epilimnion of Lake Constance [J]. Archiv für Hydrobiologie, 2001, 151(1): 157-176. doi: 10.1127/archiv-hydrobiol/151/2001/157
[36] Fetzer S, Conrad R. Effect of redox potential on methanogenesis by Methanosarcina barkeri [J]. Archives of Microbiology, 1993, 160(2): 108-113. doi: 10.1007/BF00288711
[37] Fetzer S, Bak F, Conrad R. Sensitivity of methanogenic bacteria from paddy soil to oxygen and desiccation [J]. FEMS Microbiology Ecology, 1993, 12(2): 107-115. doi: 10.1111/j.1574-6941.1993.tb00022.x
[38] Thauer R K, Kaster A K, Goenrich M, et al. Hydrogenases from methanogenic archaea, nickel, a novel cofactor, and H2 storage [J]. Annual Review of Biochemistry, 2010, 79: 507-536. doi: 10.1146/annurev.biochem.030508.152103
[39] Yuan Y L, Conrad R, Lu Y H. Transcriptional response of methanogen mcrA genes to oxygen exposure of rice field soil [J]. Environmental Microbiology Reports, 2011, 3(3): 320-328. doi: 10.1111/j.1758-2229.2010.00228.x
[40] Faber E, Berner U, Gerling P, et al. Isotopic tracing of methane in water and exchange with the atmosphere [J]. Energy Conversion and Management, 1996, 37(6-8): 1193-1198. doi: 10.1016/0196-8904(95)00319-3
[41] Bogard M J, del Giorgio P A, Boutet L, et al. Oxic water column methanogenesis as a major component of aquatic CH4 fluxes [J]. Nature Communications, 2014, 5(1): 5350. doi: 10.1038/ncomms6350
[42] Marty D, Nival P, Yoon W D. Methanoarchaea associated with sinking particles and zooplankton collected in the Northeastern tropical Atlantic [J]. Oceanologica Acta, 1997, 20(6): 863-869.
[43] Karl D M, Tilbrook B D. Production and transport of methane in oceanic particulate organic matter [J]. Nature, 1994, 368(6473): 732-734. doi: 10.1038/368732a0
[44] Sasakawa M, Tsunogai U, Kameyama S, et al. Carbon isotopic characterization for the origin of excess methane in subsurface seawater [J]. Journal of Geophysical Research, 2008, 113(C3): C03012.
[45] Oremland R S. Methanogenic activity in plankton samples and fish intestines A mechanism for in situ methanogenesis in oceanic surface waters [J]. Limnology and Oceanography, 1979, 24(6): 1136-1141. doi: 10.4319/lo.1979.24.6.1136
[46] Van Der Maarel M J E C, Sprenger W, Haanstra R, et al. Detection of methanogenic archaea in seawater particles and the digestive tract of a marine fish species [J]. FEMS Microbiology Letters, 1999, 173(1): 189-194. doi: 10.1111/j.1574-6968.1999.tb13501.x
[47] Bianchi M, Marty D, Teyssie J L, et al. Strictly aerobic and anaerobic bacteria associated with sinking particulate matter and zooplankton fecal pellets [J]. Marine Ecology Progress Series, 1992, 88: 55-60. doi: 10.3354/meps088055
[48] de Angelis M A, Lee C. Methane production during zooplankton grazing on marine phytoplankton [J]. Limnology and Oceanography, 1994, 39(6): 1298-1308. doi: 10.4319/lo.1994.39.6.1298
[49] Ditchfield A K, Wilson S T, Hart M C, et al. Identification of putative methylotrophic and hydrogenotrophic methanogens within sedimenting material and copepod faecal pellets [J]. Aquatic Microbial Ecology, 2012, 67(2): 151-160. doi: 10.3354/ame01585
[50] Angel R, Claus P, Conrad R. Methanogenic archaea are globally ubiquitous in aerated soils and become active under wet anoxic conditions [J]. The ISME Journal, 2012, 6(4): 847-862. doi: 10.1038/ismej.2011.141
[51] Angel R, Matthies D, Conrad R. Activation of methanogenesis in arid biological soil crusts despite the presence of oxygen [J]. PLoS One, 2011, 6(5): e20453. doi: 10.1371/journal.pone.0020453
[52] Grossart H P, Frindte K, Dziallas C, et al. Microbial methane production in oxygenated water column of an oligotrophic lake [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(49): 19657-19661. doi: 10.1073/pnas.1110716108
[53] Paganin P, Chiarini L, Bevivino A, et al. Vertical distribution of bacterioplankton in Lake Averno in relation to water chemistry [J]. FEMS Microbiology Ecology, 2013, 84(1): 176-188. doi: 10.1111/1574-6941.12048
[54] Lyu Z, Lu Y H. Metabolic shift at the class level sheds light on adaptation of methanogens to oxidative environments [J]. The ISME Journal, 2018, 12(2): 411-423. doi: 10.1038/ismej.2017.173
[55] Liu Y C, Whitman W B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea [J]. Annals of the New York Academy of Sciences, 2008, 1125(1): 171-189. doi: 10.1196/annals.1419.019
[56] Sieburth J M, Donaghay P L. Planktonic methane production and oxidation within the algal maximum of the pycnocline: seasonal fine-scale observations in an anoxic estuarine basin [J]. Marine Ecology Progress Series, 1993, 100: 3-15. doi: 10.3354/meps100003
[57] Tang K W, McGinnis D F, Frindte K, et al. Paradox reconsidered: methane oversaturation in well‐oxygenated lake waters [J]. Limnology and Oceanography, 2014, 59(1): 275-284. doi: 10.4319/lo.2014.59.1.0275
[58] Conrad R, Seiler W. Contribution of hydrogen production by biological nitrogen fixation to the global hydrogen budget [J]. Journal of Geophysical Research, 1980, 85(C10): 5493-5498. doi: 10.1029/JC085iC10p05493
[59] Tholen A, Pester M, Brune A. Simultaneous methanogenesis and oxygen reduction by Methanobrevibacter cuticularis at low oxygen fluxes [J]. FEMS Microbiology Ecology, 2007, 62(3): 303-312. doi: 10.1111/j.1574-6941.2007.00390.x
[60] Sprenger W W, Hackstein J H P, Keltjens J T, et al. The competitive success of Methanomicrococcus blatticola, a dominant methylotrophic methanogen in the cockroach hindgut, is supported by high substrate affinities and favorable thermodynamics [J]. FEMS Microbiology Ecology, 2007, 60(2): 266-275. doi: 10.1111/j.1574-6941.2007.00287.x
[61] Bruhn D, Mikkelsen T N, Øbro J, et al. Effects of temperature, ultraviolet radiation and pectin methyl esterase on aerobic methane release from plant material [J]. Plant Biology, 2009, 11(S1): 43-48.
[62] Ghyczy M, Torday C, Kaszaki J, et al. Hypoxia-induced generation of methane in mitochondria and eukaryotic cells-an alternative approach to methanogenesis [J]. Cellular Physiology and Biochemistry, 2008, 21(1-3): 251-258. doi: 10.1159/000113766
[63] Keppler F, Hamilton J T G, Braß M, et al. Methane emissions from terrestrial plants under aerobic conditions [J]. Nature, 2006, 439(7073): 187-191. doi: 10.1038/nature04420
[64] Lenhart K, Bunge M, Ratering S, et al. Evidence for methane production by saprotrophic fungi [J]. Nature Communications, 2012, 3(1): 1046. doi: 10.1038/ncomms2049
[65] Wang Z P, Chang S X, Chen H, et al. Widespread non-microbial methane production by organic compounds and the impact of environmental stresses [J]. Earth-Science Reviews, 2013, 127(2): 193-202.
[66] Liu J G, Chen H, Zhu Q, et al. A novel pathway of direct methane production and emission by eukaryotes including plants, animals and fungi: an overview [J]. Atmospheric Environment, 2015, 115: 26-35. doi: 10.1016/j.atmosenv.2015.05.019
[67] Keller M D, Bellows W K, Guillard R R L. Dimethyl sulfide production in marine phytoplankton[M]//Saltzman E S, Cooper W J. Biogenic Sulfur in the Environment. Washington DC: American Chemical Society, 1989: 167-182.
[68] Zindler C, Bracher A, Marandino C A, et al. Sulphur compounds, methane, and phytoplankton: interactions along a north-south transit in the western Pacific Ocean [J]. Biogeosciences Discussion, 2012, 9(10): 15011-15049. doi: 10.5194/bgd-9-15011-2012
[69] Damm E, Helmke E, Thoms S, et al. Methane production in aerobic oligotrophic surface water in the central Arctic Ocean [J]. Biogeosciences, 2010, 7(3): 1099-1108. doi: 10.5194/bg-7-1099-2010
[70] Andreae M O. Ocean-atmosphere interactions in the global biogeochemical sulfur cycle [J]. Marine Chemistry, 1990, 30: 1-29. doi: 10.1016/0304-4203(90)90059-L
[71] Taylor B F, Gilchrist D C. New routes for aerobic biodegradation of dimethylsulfoniopropionate [J]. Applied and Environmental Microbiology, 1991, 57(12): 3581-3584.
[72] Kiene R P, Oremland R S, Catena A, et al. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen [J]. Applied and Environmental Microbiology, 1986, 52(5): 1037-1045.
[73] Finster K, Tanimoto Y, Bak F. Fermentation of methanethiol and dimethylsulfide by a newly isolated methanogenic bacterium [J]. Archives of Microbiology, 1992, 157(5): 425-430. doi: 10.1007/BF00249099
[74] Karl D M, Beversdorf L, Björkman K M, et al. Aerobic production of methane in the sea [J]. Nature Geoscience, 2008, 1(7): 473-478. doi: 10.1038/ngeo234
[75] Villarreal-Chiu J F, Quinn J P, Mcgrath J W. The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment [J]. Frontiers in Microbiology, 2012, 3: 19.
[76] Wang Q, Dore J E, McDermott T R. Methylphosphonate metabolism by Pseudomonas sp. populations contributes to the methane oversaturation paradox in an oxic freshwater lake [J]. Environmental Microbiology, 2017, 19(6): 2366-2378. doi: 10.1111/1462-2920.13747
[77] Carini P, White A E, Campbell E O, et al. Methane production by phosphate-starved SAR11 chemoheterotrophic marine bacteria [J]. Nature Communications, 2014, 5(1): 4346. doi: 10.1038/ncomms5346
[78] Metcalf W W, Griffin B M, Cicchillo R M, et al. Synthesis of methylphosphonic acid by marine microbes: a source for methane in the aerobic ocean [J]. Science, 2015, 337(6098): 1104-1107.
[79] Karner M B, DeLong E F, Karl D M. Archaeal dominance in the mesopelagic zone of the Pacific Ocean [J]. Nature, 2001, 409(6819): 507-510. doi: 10.1038/35054051
[80] Könneke M, Bernhard A E, de la Torre J R, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon [J]. Nature, 2005, 437(7058): 543-546. doi: 10.1038/nature03911
[81] Kolowith L C, Ingall E D, Benner R. Composition and cycling of marine organic phosphorus [J]. Limnology and Oceanography, 2001, 46(2): 309-320. doi: 10.4319/lo.2001.46.2.0309
[82] Sannigrahi P, Ingall E D, Benner R. Cycling of dissolved and particulate organic matter at station Aloha: insights from 13C NMR spectroscopy coupled with elemental, isotopic and molecular analyses [J]. Deep Sea Research Part I: Oceanographic Research Papers, 2005, 52(8): 1429-1444. doi: 10.1016/j.dsr.2005.04.001
[83] Santoro A E, Dupont C L, Richter R A, et al. Genomic and proteomic characterization of “Candidatus Nitrosopelagicus brevis”: an ammonia-oxidizing archaeon from the open ocean [J]. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(4): 1173-1178. doi: 10.1073/pnas.1416223112
[84] Del Valle D A, Karl D M. Aerobic production of methane from dissolved water-column methylphosphonate and sinking particles in the North Pacific Subtropical Gyre [J]. Aquatic Microbial Ecology, 2014, 73(2): 93-105. doi: 10.3354/ame01714
[85] Repeta D J, Ferrón S, Sosa O A, et al. Marine methane paradox explained by bacterial degradation of dissolved organic matter [J]. Nature Geoscience, 2016, 9(12): 884-887. doi: 10.1038/ngeo2837
[86] Yu X M, Doroghazi J R, Janga S C, et al. Diversity and abundance of phosphonate biosynthetic genes in nature [J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(51): 20759-20764. doi: 10.1073/pnas.1315107110
[87] Gomez-Garcia M R, Davison M, Blain-Hartnung M, et al. Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats [J]. The ISME Journal, 2011, 5(1): 141-149. doi: 10.1038/ismej.2010.96
[88] Scranton M I, Farrington J W. Methane production in the waters off Walvis Bay [J]. Journal of Geophysical Research, 1977, 82(31): 4947-4953. doi: 10.1029/JC082i031p04947
[89] Scranton M I, Brewer P G. Occurrence of methane in the near-surface waters of the western subtropical North-Atlantic [J]. Deep Sea Research, 1977, 24(2): 127-138. doi: 10.1016/0146-6291(77)90548-3
[90] Lenhart K, Klintzsch T, Langer G, et al. Evidence for methane production by the marine algae Emiliana huxleyi [J]. Biogeosciences Discussions, 2015, 12(24): 20323-20360. doi: 10.5194/bgd-12-20323-2015
[91] Althoff F, Jugold A, Keppler F. Methane formation by oxidation of ascorbic acid using iron minerals and hydrogen peroxide [J]. Chemosphere, 2010, 80(3): 286-292. doi: 10.1016/j.chemosphere.2010.04.004
[92] Althoff F, Benzing K, Comba P, et al. Abiotic methanogenesis from organosulphur compounds under ambient conditions [J]. Nature Communications, 2014, 5(1): 4205. doi: 10.1038/ncomms5205
[93] Bange H W, Uher G. Photochemical production of methane in natural waters: implications for its present and past oceanic source [J]. Chemosphere, 2005, 58(2): 177-183. doi: 10.1016/j.chemosphere.2004.06.022
[94] 耿澜涛. 加拿大北极亚北极海水中溶解甲烷的分布及其生物地球化学研究[D]. 中国地质大学博士学位论文, 2017. GENG Lantao. Studies on the distribution of dissolved methane and its biogeochemistry in Canadian Arctic and sub-Arctic Seas[D]. Doctor Dissertation of China University of Geosciences, 2017.
[95] Bižić-Ionescu M, Ionescu D, Günthel M, et al. Oxic methane cycling: new evidence for methane formation in oxic lake water[M]//Stams A J M, Sousa D. Biogenesis of Hydrocarbons. Cham: Springer, 2018: 1-22.
[96] Ward B B, Kilpatrick K A, Novelli P C, et al. Methane oxidation and methane fluxes in the ocean surface layer and deep anoxic waters [J]. Nature, 1987, 327(6119): 226-229. doi: 10.1038/327226a0
[97] Pack M A, Heintz M B, Reeburgh W S, et al. Methane oxidation in the eastern tropical North Pacific Ocean water column [J]. Journal of Geophysical Research, 2015, 120(6): 1078-1092.
[98] Murase J, Sugimoto A. Inhibitory effect of light on methane oxidation in the pelagic water column of a mesotrophic lake (Lake Biwa, Japan) [J]. Limnology and Oceanography, 2005, 50(4): 1339-1343. doi: 10.4319/lo.2005.50.4.1339
[99] Thauer R K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson: 1998 Marjory Stephenson Prize Lecture [J]. Microbiology, 1998, 144(9): 2377-2406. doi: 10.1099/00221287-144-9-2377
[100] Welte C, Deppenmeier U. Bioenergetics and anaerobic respiratory chains of aceticlastic methanogens [J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2014, 1837(7): 1130-1147. doi: 10.1016/j.bbabio.2013.12.002
[101] Costa K C, Leigh J A. Metabolic versatility in methanogens [J]. Current Opinion in Biotechnology, 2014, 29: 70-75. doi: 10.1016/j.copbio.2014.02.012
[102] Ermler U, Grabarse W, Shima S, et al. Crystal structure of methyl-coenzyme M reductase: the key enzyme of biological methane formation [J]. Science, 1997, 278(5342): 1457-1462. doi: 10.1126/science.278.5342.1457
[103] Scheller S, Goenrich M, Boecher R, et al. The key nickel enzyme of methanogenesis catalyses the anaerobic oxidation of methane [J]. Nature, 2010, 465(7298): 606-608. doi: 10.1038/nature09015
[104] Lueders T, Chin K J, Conrad R, et al. Molecular analyses of methyl-coenzyme M reductase α-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage [J]. Environmental Microbiology, 2001, 3(3): 194-204. doi: 10.1046/j.1462-2920.2001.00179.x
[105] Imlay J A. Cellular defenses against superoxide and hydrogen peroxide [J]. Annual Review of Biochemistry, 2008, 77: 755-776. doi: 10.1146/annurev.biochem.77.061606.161055
[106] 承磊, 郑珍珍, 王聪, 等. 产甲烷古菌研究进展[J]. 微生物学通报, 2016, 43(5):1143-1164. [CHENG Lei, ZHENG Zhenzhen, WANG Cong, et al. Recent advances in methanoges [J]. Microbiology China, 2016, 43(5): 1143-1164. -
期刊类型引用(3)
1. 陈建文,孙晶,杨长清,杨传胜,谢明英,孙晓娜,王建强,袁勇,曹珂. 东海陆架盆地新生界咸水层二氧化碳封存地质条件及封存前景. 海洋地质前沿. 2023(10): 14-21 .  百度学术
百度学术
2. 李青,陈建洲,王国仓,王瑾,谢菁,王琪玮,晁海德. 青藏高原北部东昆仑地区三叠系元素地球化学组成对古环境的指示意义. 天然气地球科学. 2021(11): 1714-1723 .  百度学术
百度学术
3. 尚鲁宁,张勇,姚永坚,吴浩,胡刚,田陟贤. 中国东部大陆边缘晚新生代构造演化及板块相互作用过程重建. 中国地质. 2020(05): 1323-1336 .  百度学术
百度学术
其他类型引用(0)




 下载:
下载: